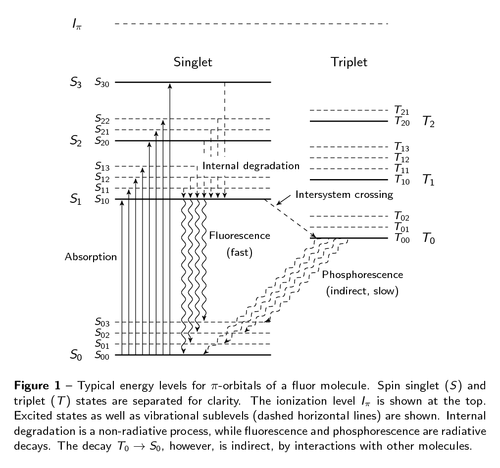
In particle physics, the most important experimental problem is that of detecting particles. A cheap and highly efficient solution is using scintillators. These kinds of detectors emit light when a charged particle traverses the detector. The light-emitting process is depicted in this figure. The layout was inspired by [this wikipedia graphic](http://en.wikipedia.org/wiki/File:Pistates.svg). The TikZ code in my version is a bit complex, mainly due to manual layout tweaks, shifting some positions here and there. The figure shown here is a minor revision of the one included in [my PhD thesis](http://dx.doi.org/10.3990/1.9789036534383).
Edit and compile if you like:
% Energy levels of a fluor molecule
% Author: David Fokkema
\documentclass{article}
\usepackage{tikz}
\usepackage[active,tightpage]{preview}
\PreviewEnvironment{center}
\setlength\PreviewBorder{10pt}%
\usetikzlibrary{calc,arrows,decorations.pathmorphing,intersections}
\usepackage[font={small,sf},labelfont={bf},labelsep=endash]{caption}
\usepackage{sansmath}
\begin{document}
\begin{center}
\sansmath
\begin{tikzpicture}[
font=\sffamily,
level/.style={black,thick},
sublevel/.style={black,densely dashed},
ionization/.style={black,dashed},
transition/.style={black,->,>=stealth',shorten >=1pt},
radiative/.style={transition,decorate,decoration={snake,amplitude=1.5}},
indirectradiative/.style={radiative,densely dashed},
nonradiative/.style={transition,dashed},
]
\coordinate (sublevel) at (0, 8pt);
% Singlet levels
\coordinate (S00) at (0, -1);
\coordinate (S01) at ($(S00) + (sublevel)$);
\coordinate (S02) at ($(S00) + 2*(sublevel)$);
\coordinate (S03) at ($(S00) + 3*(sublevel)$);
\coordinate (S10) at (0, 3);
\coordinate (S11) at ($(S10) + (sublevel)$);
\coordinate (S12) at ($(S10) + 2*(sublevel)$);
\coordinate (S13) at ($(S10) + 3*(sublevel)$);
\coordinate (S20) at (0, 4.5);
\coordinate (S21) at ($(S20) + (sublevel)$);
\coordinate (S22) at ($(S20) + 2*(sublevel)$);
\coordinate (S30) at (0, 6);
% Draw main levels
\foreach \level/\text in {00/0, 10/1, 20/2, 30/3}
\draw[level] (S\level) node[left=20pt] {$S_\text$} node[left]
{\footnotesize $S_{\level}$} -- +(4, 0);
% Draw sublevels
\foreach \sublevel in {01,02,03,11,12,13,21,22}
\draw[sublevel] (S\sublevel) node[left]
{\footnotesize $S_{\sublevel}$} -- +(4, 0);
\node at (2, 6.5) {Singlet};
% Triplet levels
\coordinate (T00) at (5, 2);
\coordinate (T01) at ($(T00) + (sublevel)$);
\coordinate (T02) at ($(T00) + 2*(sublevel)$);
\coordinate (T03) at ($(T00) + 3*(sublevel)$);
\coordinate (T10) at (5, 3.5);
\coordinate (T11) at ($(T10) + (sublevel)$);
\coordinate (T12) at ($(T10) + 2*(sublevel)$);
\coordinate (T13) at ($(T10) + 3*(sublevel)$);
\coordinate (T20) at (5, 5);
\coordinate (T21) at ($(T20) + (sublevel)$);
% Draw main levels
\foreach \level/\text in {00/0, 10/1, 20/2}
\draw[level] (T\level) -- +(2, 0)
node[right=20pt] {$T_\text$}
node[right] {\footnotesize $T_{\level}$};
% Draw sublevels
\foreach \sublevel in {01,02,11,12,13,21}
\draw[sublevel] (T\sublevel) -- +(2, 0) node[right]
{\footnotesize $T_{\sublevel}$};
\node at (6, 6.5) {Triplet};
% Ionization level
\draw[ionization] (0, 7.5) node[left=20pt] {$I_\pi$} -- +(7, 0);
% Excitations
\foreach \i/\from/\to in {1/S00/S10, 2/S00/S11, 3/S00/S12, 4/S00/S13,
5/S00/S20, 6/S00/S21, 7/S00/S22, 8/S00/S30}
\draw[transition] ([xshift=\i*5pt] \from) -- ([xshift=\i*5pt] \to);
% Radiative decay (fluorescence)
\foreach \i/\from/\to in {1/S10/S00, 2/S10/S01, 3/S10/S02, 4/S10/S03}
\draw[radiative] ([xshift=(\i+9)*5pt] \from) --
([xshift=(\i+9)*5pt] \to);
% Nonradiative decay (internal degradation)
\foreach \i/\from/\to in {1/S11/S10, 2/S12/S10, 3/S13/S10, 4/S20/S10,
5/S21/S10, 6/S22/S10, 7/S30/S10}
\draw[nonradiative] ([xshift=(\i+9)*5pt] \from) --
([xshift=(\i+9)*5pt] \to);
% Radiative decay (phosphorescence)
%
% There is some magic going on to prevent an irritating optical effect.
% If the (start) coordinate is taken to be simply (Tstart), the wiggly
% lines start at the T00 level. Because of their differing lengths
% however, the wiggles start to form a distracting pattern. Therefore,
% the lines are extended a bit (-\i*5pt) to show a pleasing effect. They
% are clipped so the transition still starts at T00. If you want to
% observe the optical effect, include this line at the correct location:
% \coordinate (start) at (Tstart);
\begin{scope}
\clip (S00) -- +(7, 0) |- (T00) -| (S00);
\foreach \i/\level in {1/(S00), 2/(S01), 3/(S02), 4/(S03)} {
\coordinate (Tstart) at ([xshift=\i*7pt] T00);
\coordinate (end) at ($(Tstart) + (-135:4.5)$);
\coordinate (start) at ($(Tstart)!-\i*5pt!(end)$);
\path[name path=trans] (start) -- (end);
\path[name path=ground] \level -- +(5, 0);
\draw[indirectradiative,name intersections={of=trans and ground}]
(start) -- (intersection-1);
}
\end{scope}
% Labels (curious coordinates are due to manual placement adjustments)
\node[left] at (5pt, 1.5) {\footnotesize Absorption};
\node[right,align=center] at (13*5pt, 2cm - 5pt)
{\footnotesize Fluorescence\\\footnotesize (fast)};
\node[right,align=center] at (5cm + 5pt, 1cm - 5pt)
{\footnotesize Phosphorescence\\\footnotesize (indirect, slow)};
\node[right,fill=white,align=left] at ([xshift=12*5pt] S13)
{\footnotesize Internal degradation};
% Intersystem crossing
\draw[nonradiative,name path=crossing] ($(S10) + (4, 0) - (5pt, 0)$) --
([xshift=5pt] T00);
\coordinate (crosslabel) at (4.5, 3.1);
\node[right,fill=white] at (crosslabel) {\footnotesize Intersystem crossing};
\path[name path=arrow] (crosslabel) -- +(-145:1cm);
\draw[->,>=stealth',shorten >=2pt,
name intersections={of=crossing and arrow}]
(crosslabel) -- (intersection-1);
\end{tikzpicture}
\captionof{figure}{Typical energy levels for $\pi$-orbitals of a fluor
molecule. Spin singlet~($S$) and triplet~($T$) states are separated for
clarity. The ionization level $I_\pi$ is shown at the top. Excited states
as well as vibrational sublevels (dashed horizontal lines) are shown.
Internal degradation is a non-radiative process, while fluorescence and
phosphorescence are radiative decays. The decay $T_0 \to S_0$, however,
is indirect, by interactions with other molecules.}
\end{center}
\end{document}
Click to download: fluor-energy-levels.tex • fluor-energy-levels.pdf
Open in Overleaf: fluor-energy-levels.tex


