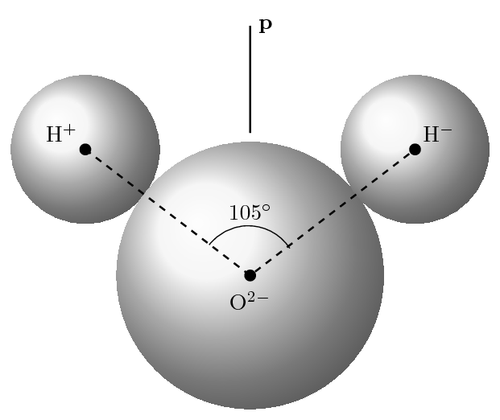
The diagram shows the geometry of hydrogen and oxygen atoms in the water molecule, and the position of the dipole (p). Using known dimensions, we can calculate the energy of the dipole, and, consequently, the interaction energy between two molecules.
Edit and compile if you like:
% The electric dipole moment (p) in the water molecule
% Author: Jimi Oke
\documentclass{article}
\usepackage{tikz}
\usepackage[active,tightpage]{preview}
\PreviewEnvironment{tikzpicture}
\setlength\PreviewBorder{5pt}%
\begin{document}
\begin{tikzpicture}[>=latex,scale=1.3]
\shade[ball color=gray!10!] (0,0) coordinate(Hp) circle (.9) ;
\shade[ball color=gray!10!] (2,-1.53) coordinate(O) circle (1.62) ;
\shade[ball color=gray!10!] (4,0) coordinate(Hm) circle (.9) ;
\draw[thick,dashed] (0,0) -- (2,-1.53) -- (4,0) ;
\draw[thick] (2,.2) -- (2,1.5) node[right]{$\mathbf{p}$} ;
\draw (2.48,-1.2) arc (33:142:.6) ;
\draw (2,-.95) node[above]{$105^{\circ}$} ;
\draw (0,.2) node[left]{H$^+$} ;
\draw (4,.2) node[right]{H$^-$} ;
\draw (2,-1.63) node[below]{O$^{2-}$} ;
\foreach \point in {O,Hp,Hm}
\fill [black] (\point) circle (2pt) ;
\end{tikzpicture}
\end{document}
Click to download: electric-dipole.tex • electric-dipole.pdf
Open in Overleaf: electric-dipole.tex


